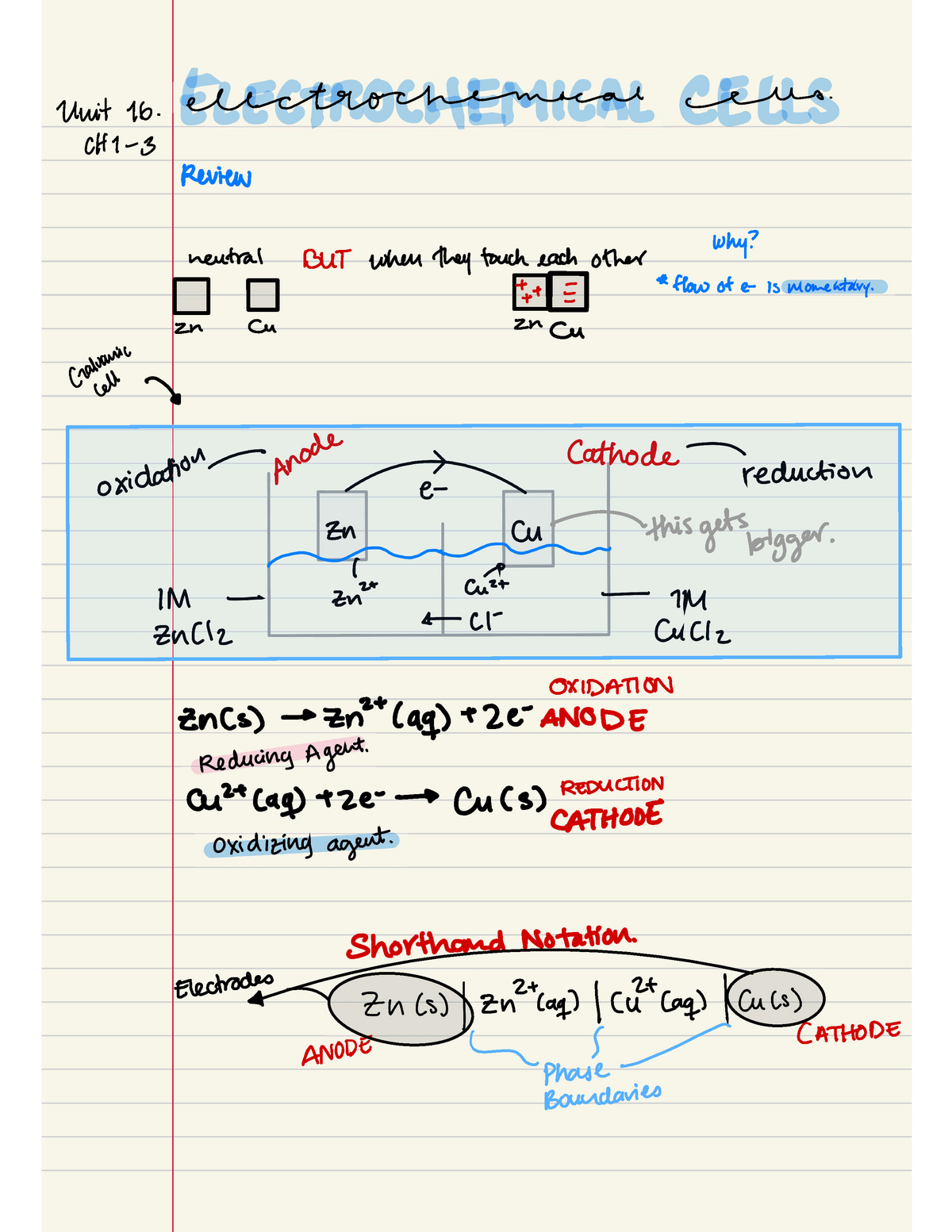
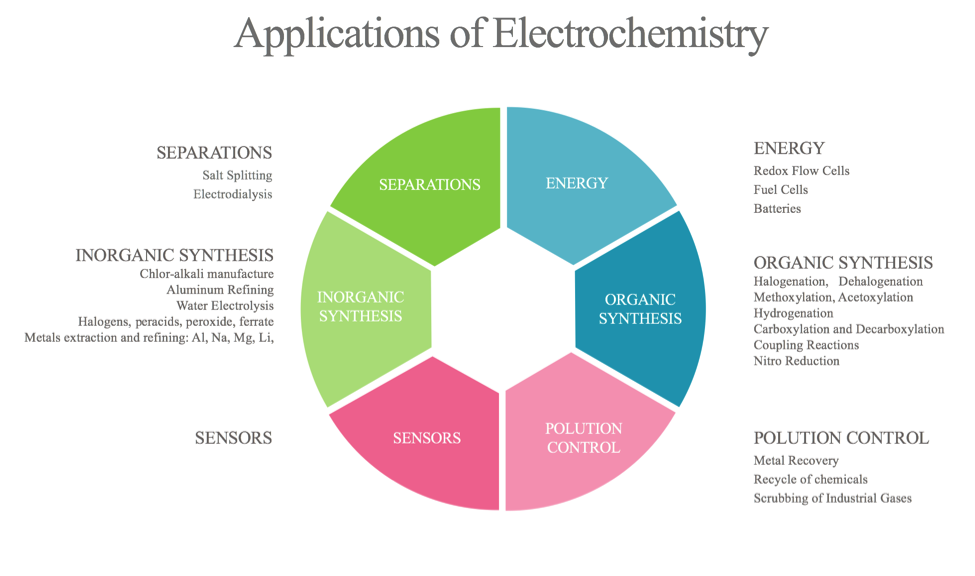
Class 12 electrochemistry revision notes for neet:Learners at any stage of their preparation will be be.
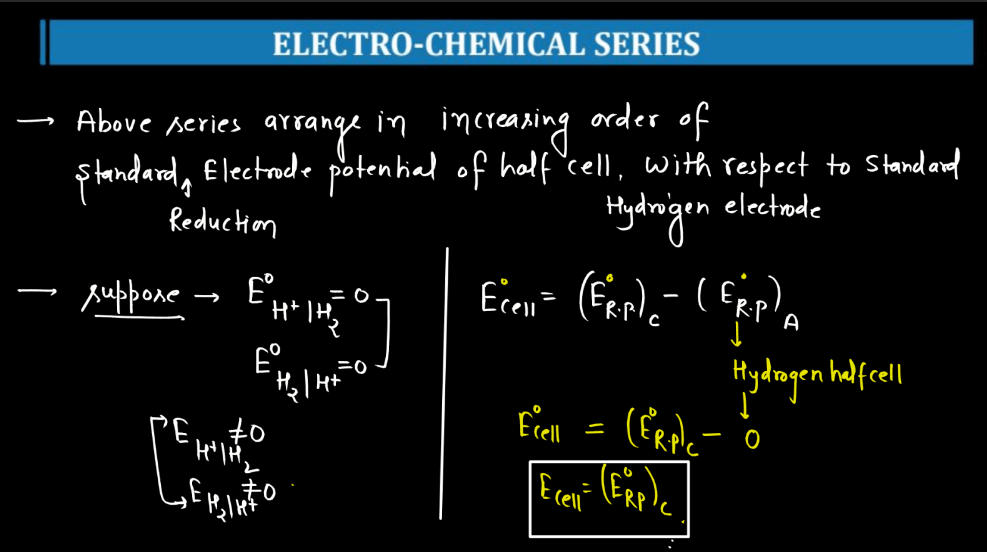
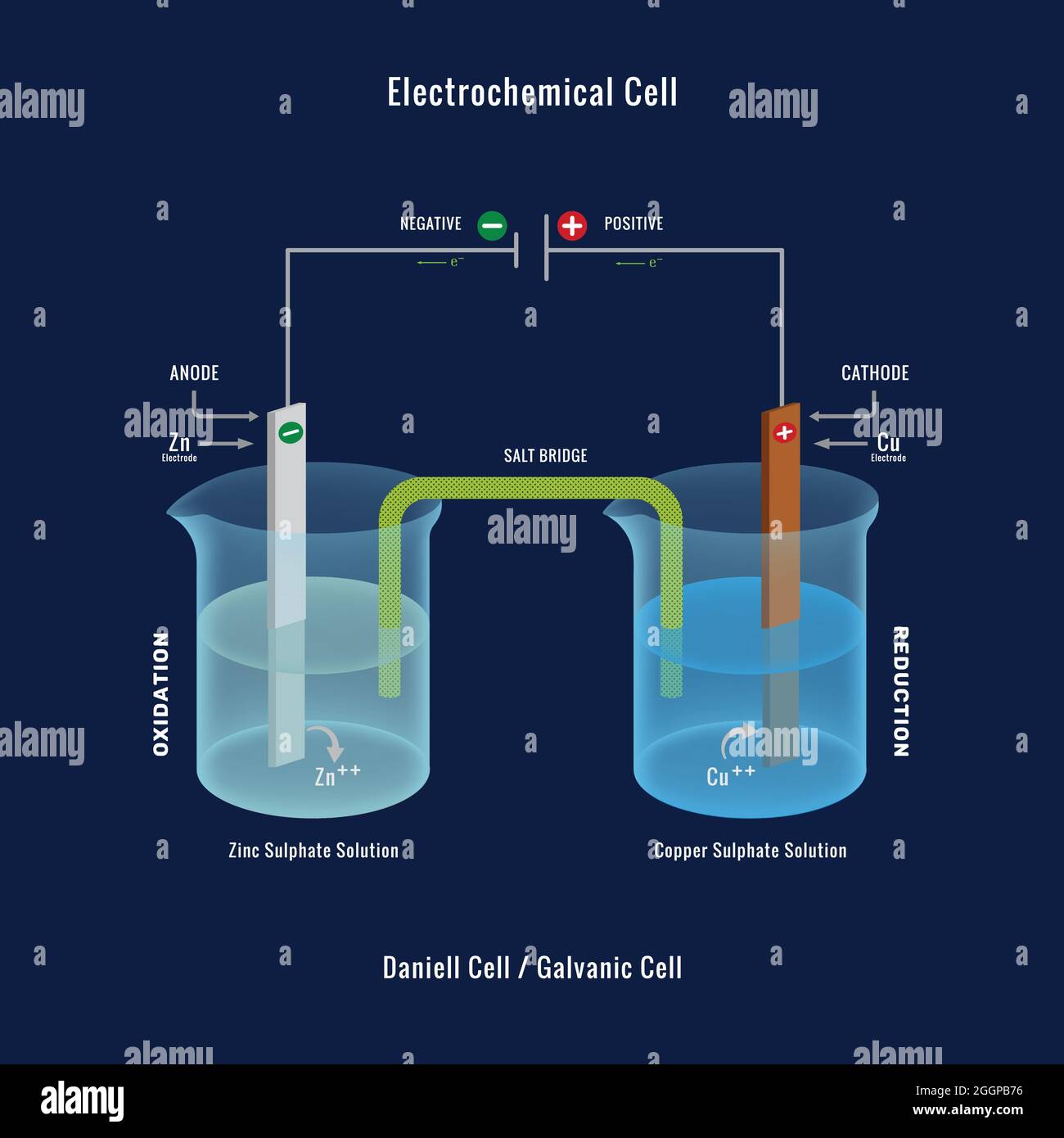
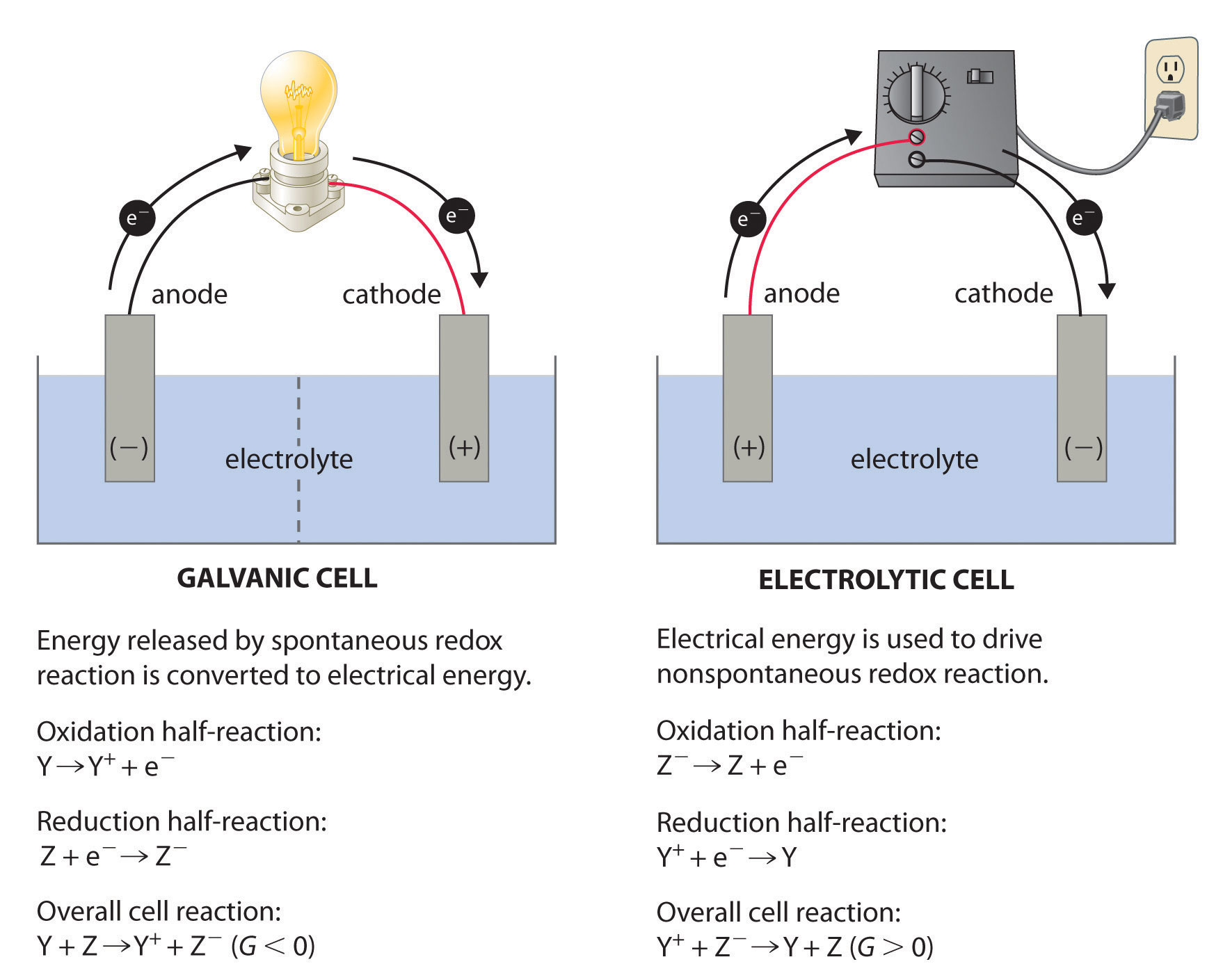
These are devices that convert the chemical energy of some redox reactions to electrical energy.they are also called galvanic cells or voltaic cells.They will also study the application of the nernst equation for calculating the emf of a galvanic cell and define the standard potential of the cell.Glucose is a highly crucial energy storage molecule considered indispensable to most living cells (bi et al., 2020).due to its polar nature and size, it is incapable of direct diffusion into the nonpolar plasma membrane (teymourian et al., 2020).however, to combat this and fulfill cellular energy requirements, glucose entry into cells is regulated by a family of structurally.
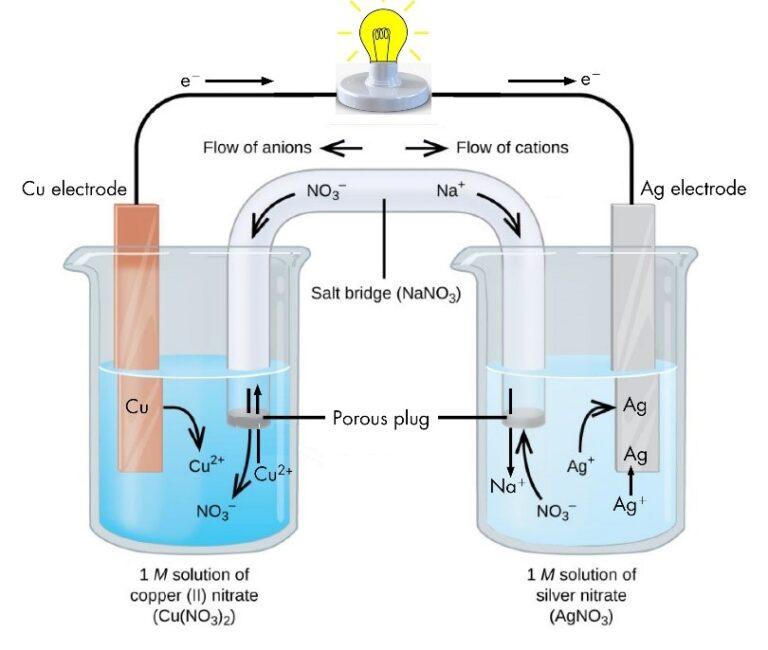
The course will be helpful for aspirants preparing for cbse class 12 exam.The other beaker contains a solution of sn2 + in dilute sulfuric acid, also with a pt electrode.
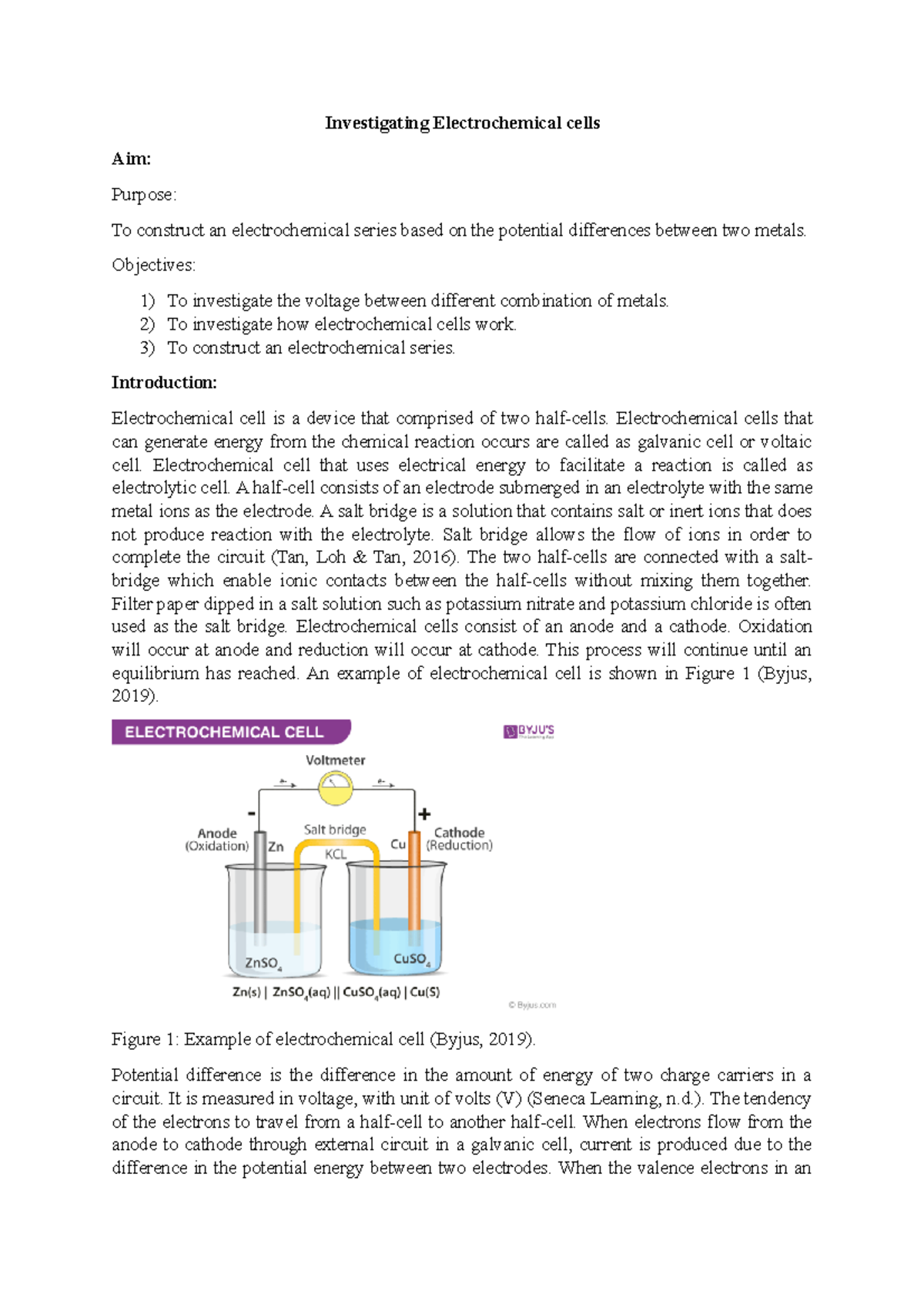
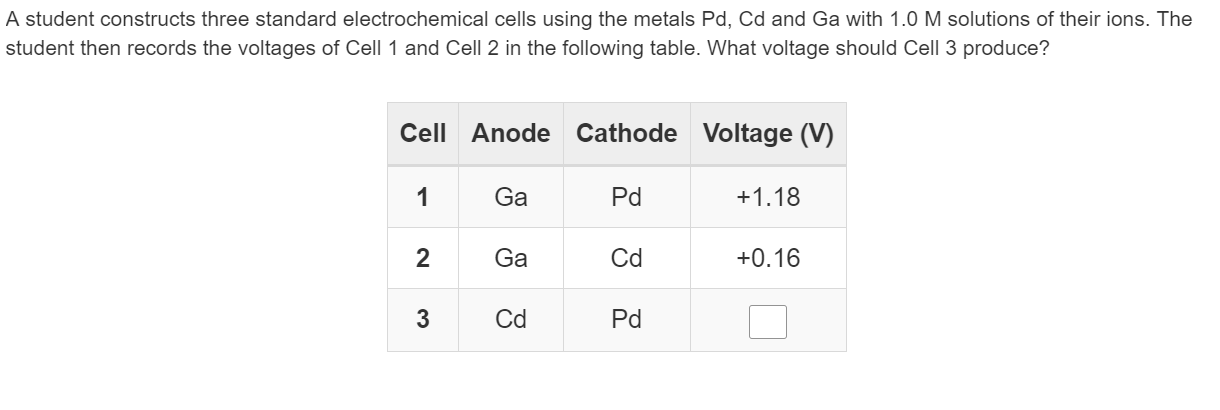
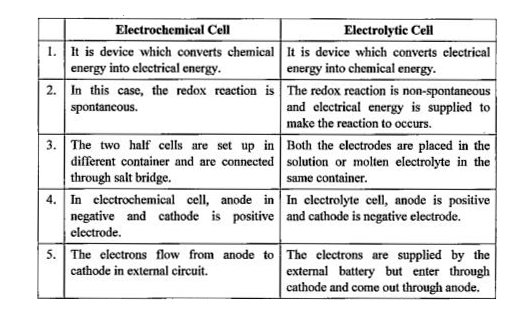
This chapter will explain what electrochemical cells are used for and how the constituents get separated using.Learn the concepts and applications of electrochemical cells, electrolysis, conductance and more.A schematic diagram of a typical electrochemical cell is shown in figure 22.1.1.
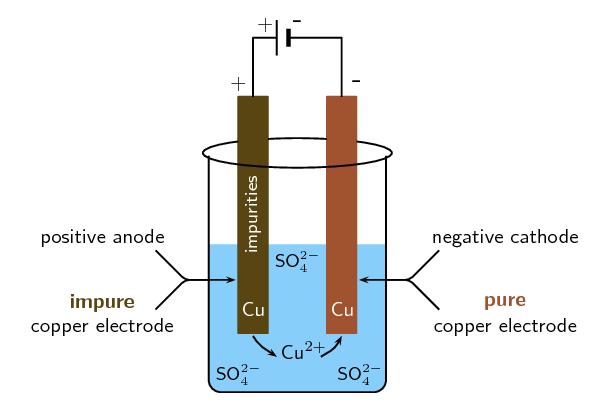
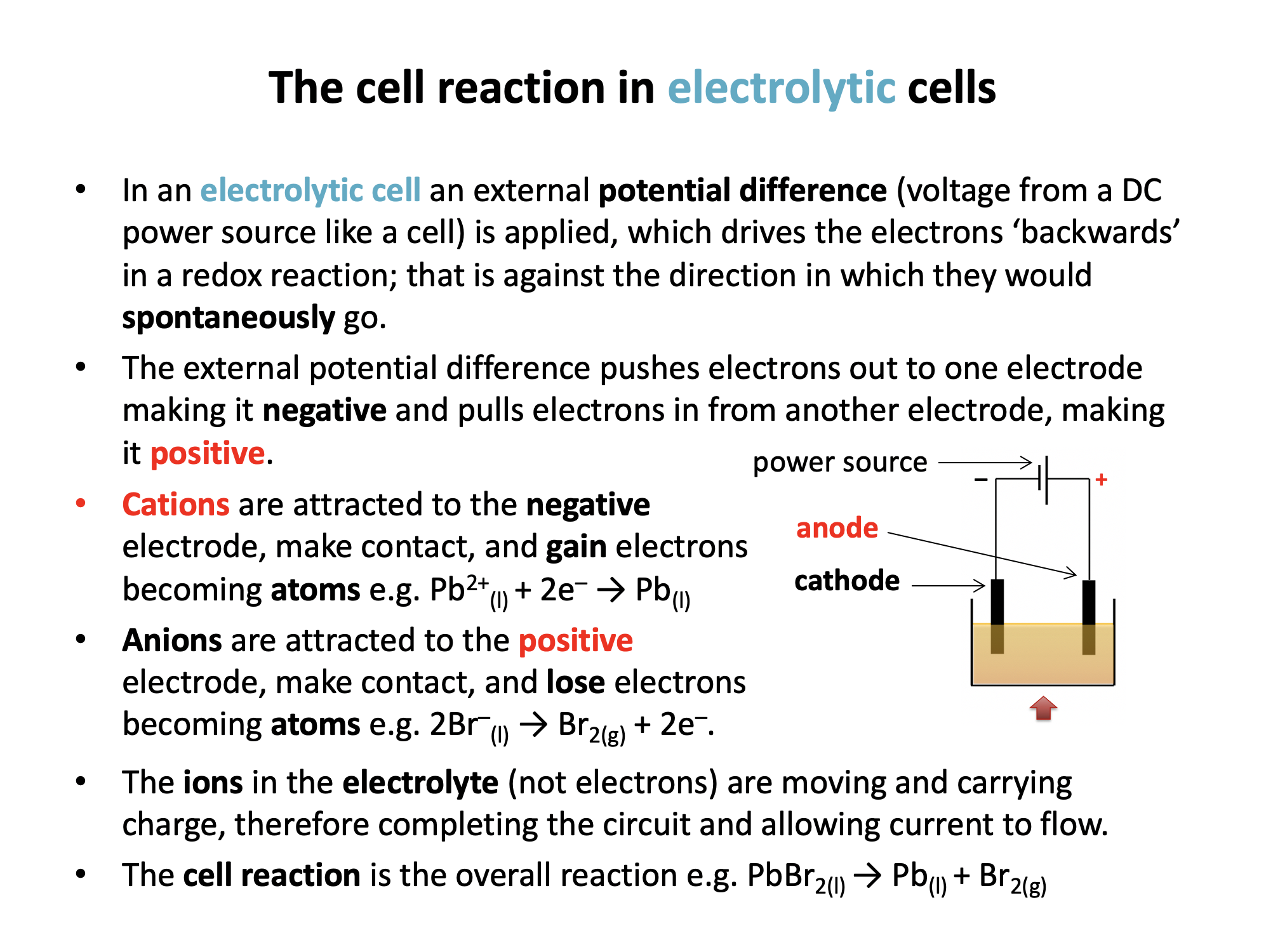
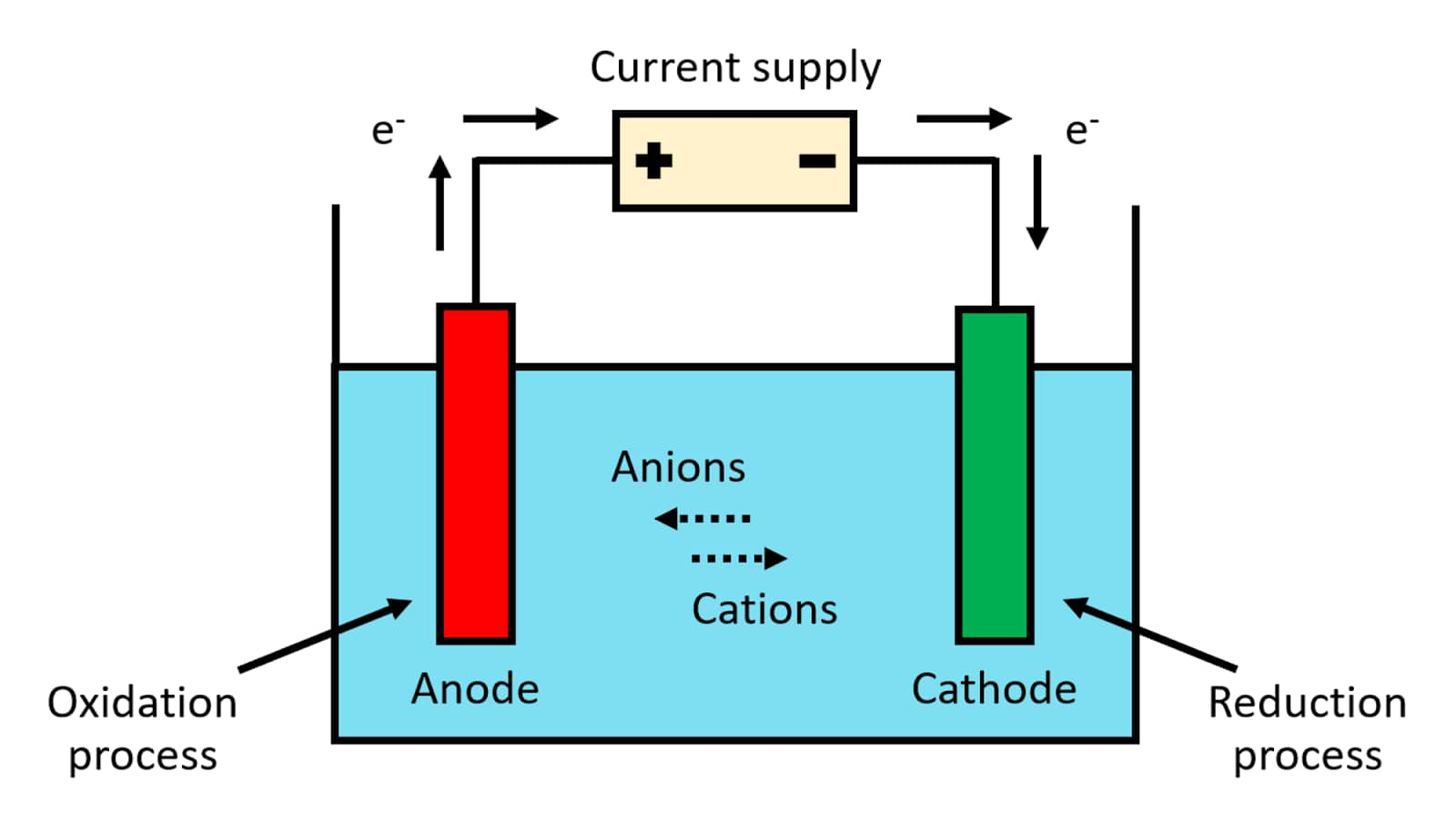
This chapter teaches students how chemical reactions occur during electrolysis.A common example of an electrochemical cell is a.
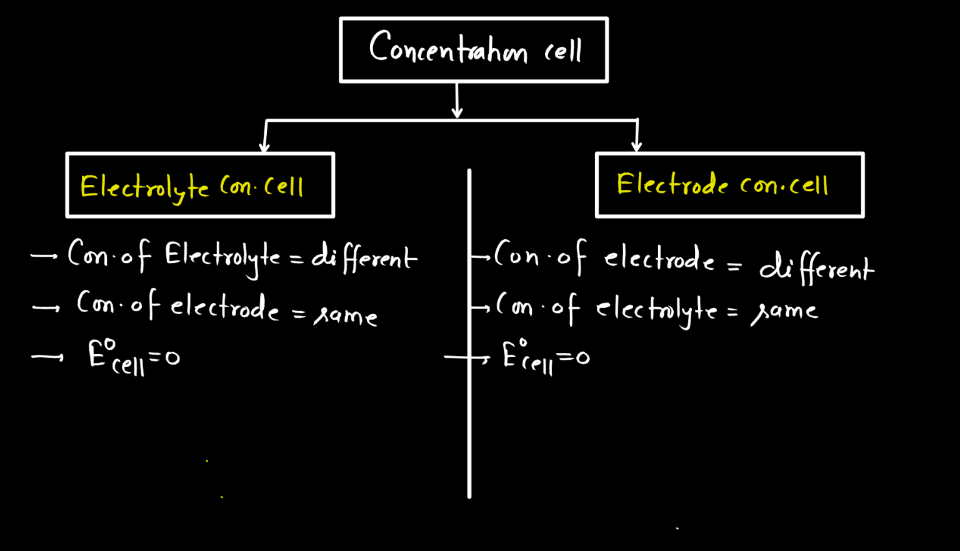
A galvanic cell is a device that turns chemical energy into electrical energy.The two solutions are connected externally by a metallic wire through a voltmeter and.Electrochemistry is a crucial part of the class 12 chemistry syllabus where students learn what electrochemical cells are and the functions of their components.
Last update images today What Is Electrochemical Cell Class 12
 Retiring Kroos Pens Emotional Post After Euro Exit
Retiring Kroos Pens Emotional Post After Euro Exit
PARIS -- Faith Kipyegon of Kenya broke her own world record in the women's 1,500 meters at the Diamond League track and field meeting in Paris on Sunday.
Kipyegon finished in 3:49.04, surpassing her record of 3:49.11, which was set in Italy last year.
"I knew the world record was possible because I recently ran very fast in Kenya," said Kipyegon, who clocked 3:53.98 at Kenya's Olympic trials. "I was coming here to just run my race and to see what shape I'm in to defend my title at the Olympics."
Nine other runners in the race achieved personal bests. Jessica Hull of Australia finished second in 3:50.83, smashing her own Oceania record by five seconds. Laura Muir was third in a British record of 3:53.79.
Kipyegon, 30, is a two-time Olympic gold medalist in the 1,500, having won in Rio de Janeiro in 2016 and Tokyo in 2021. Before Sunday, she had only run twice in 2024, in the 1,500 and 5,000, to secure her spot for the Paris Olympics at the Kenyan trials in June.
Kipyegon's performance came less than an hour after Ukraine's Yaroslava Mahuchikh broke the world high jump record with a leap of 2.10 meters.